Antibiotic arguments
DG Sanco is coming to the end of their review of antimicrobial use in the EU, shortly, what will be their recommendations?
There has been a lot of activity in the Netherlands over antimicrobial use and the demand to reduce use by 50% over the next two years and to ban their in-feed use. Let’s look at some of their problems that they have and see if their solutions might be a guide to DG Sanco. In a survey of isolates of bacteria from various food producing species taken in 2008 (Maran 2008, 2010) they have carried out minimum inhibitory concentration (MIC) tests to determine the antimicrobial resistance of various bacteria to the different antimicrobials. The important antimicrobial drugs, which are considered ‘critical’ in both human (h) and veterinary (v) medicine, are the fluoroquinolones (enrofloxacin (v), ciprofloxacin (h)) and the 3rd and 4th generation cephalosporins (ceftiofur (v), ceftazidime (h) 3rd gen). Escherichia coli is a useful bacterium that occurs in all animal species and acts as an ‘indicator’ organism for demonstrating exposure to many antimicrobials but especially these critical ones of human and animal interest (see Figure 1 and 2). The susceptibility pattern frequently tells you what is going on – how much exposure is there to an antibiotic.
Fluoroquinolones
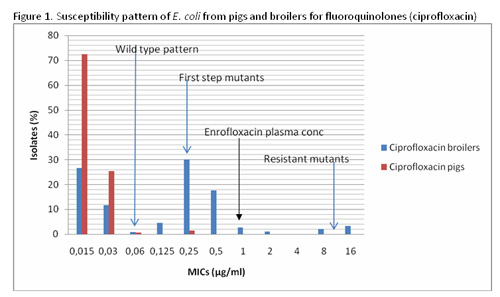
The porcine MICs on the left are very low and primarily cause a ‘wild type pattern’ up to 0.06µg/ml (98.6%). There is a small bar at 0.25µg/ml where 1.4% of the isolates show a minor first step resistance mutation but there are no fully resistant mutants. All of these isolates from pigs would respond to therapy with a fluoroquinolone and can be considered below the ‘clinical breakpoint’ for the drug or below the concentration in the plasma/blood to attack the bug. This has no impact on human health from a potential resistance transfer, even if the bacteria were eaten somehow on contaminated meat.
In contrast, in broilers there is a high level of first-step mutants (55.6%) and even some fully resistant strains (6.5%) above the clinical breakpoint, which could not be treated with this drug. This does show that the fluoroquinolones, which are excellent antimicrobial drugs, are very widely used in broilers but not in pigs. Therefore should they be banned from oral use in all chickens as in the US?
This is a useful example to highlight some of the problems DG Sanco and the Dutch will face. There are no feed premixes approved containing ciprofloxacin or enrofloxacin in the EU, so banning feed premixes will have no effect on this problem at all. They are used in broilers mainly in the drinking water and in pigs by injection and there is an oral-dosing form for baby pigs. It would appear to be causing very few problems in pigs and it is a very valuable drug for key, potentially fatal infections e.g. neonatal E. coli scours in the first few days of life and also for acute Actinobacillus pleuropneumoniae infections.
Cephalosporins 3rd & 4th generation
The susceptibility patterns are less marked with the 3rd generation cephalosporins than the fluoroquinolones, but it does demonstrate that E. coli from pigs all fall in the susceptible range but 98.7% fall in the wild type pattern. Again this poses no threat of resistance transfer to man of extended-spectrum beta-lactamase (ESBL) resistance genes. There has been methicillin-resistant Staphylococcus aureus (MRSA) resistance associated with 3rd generation cephalosporin use in pigs but ESBL resistance in E. coli would appear not to be an issue.
In broilers, somewhat surprisingly, there is a degree of resistance with 14.5% of isolates demonstrating higher than wild type pattern MICs and 6.7% above the susceptibility breakpoint used in the report. It was reported that they were ‘potentially producing ESBLs’ but this had not been confirmed.
This is another useful example, as ceftiofur (3rd generation cephalosporin) is primarily used by injection in pigs. There are no in feed or drinking water formulations. It is not even indicated for use in poultry, although it is used ‘off label’ by in-ovo injection or injection in day old chicks.
So what will DG Sanco recommend? Banning these critical families of drugs from veterinary use will rob the animal health industry of two very important groups of compounds for treating disease. Surprisingly, pigs are not a source of fluoroquinolone and ESBL resistance, which could be transferred to man in the Netherlands. The banning of in-feed use will have no impact on these resistance issues as there are no in-feed products. It will just put more pressure on water soluble and injectable products like the fluoroquinolones and cephalosporins, which should be reserved for ‘last resort’ treatments rather than routine applications. The ‘off-label’ use of ceftiofur in chickens might be an area, which could be easily addressed. The extensive use of fluoroquinolones in broilers does appear to be an issue to review further and encourage more ‘prudent use’ programmes. The other concern is that the report showed the zoonotic, food-poisoning infection associated with Campylobacter jejuni mainly from broilers, over 55.5% of isolates had MICs above ‘wild type’ susceptibility patterns against fluoroquinolones, similar to the E. coli results. As most broiler carcasses are contaminated with Campylobacter after processing this could lead to some concern that the resistance could be spread to man, although the diarrhoea problems alone would be bad enough. One simple solution would be for EFSA to implement proper carcass hygiene controls on broilers in the EU and then you would get rid of Campylobacter and the resistance issue in one hit but then we would not be able to have any arguments about antibiotics.
I wish you all a happy and festive season.











