Seneca Valley virus: What is known?
The emergence of Seneca Valley virus (SVV) and increased incidences of SVV-associated vesicular disease alarmed the US swine industry. On the plus side, this gave the opportunity to gain a better understanding of the pathogenesis, ecology and immunobiology of this lesser-known disease.
Seneca Valley virus (SVV) is a small non-enveloped virus with a positive-sense, single-stranded RNA genome of approximately 7.2 kilobases. As is the case for all RNA viruses, a relatively high rate of genetic mutation should be expected for SVV over time. Taxonomically, SVV belongs to and is the only member of the genus Senecavirus in the family Picornaviridae.
The family Picornaviridae also has several important swine pathogens, including:
- foot-and-mouth disease virus (FMDV),
- swine vesicular disease virus (SVDV),
- porcine teschovirus, encephalomyocarditis virus and
- enterovirus G.
In literature, SVV and senecavirus A (SVA) have been interchangeably used as the virus name. ‘Seneca Valley virus’ should be the virus name to be used, as Senecavirus A is the species name for the virus.
SVV was first discovered serendipitously in 2002 as a cell culture contaminant, named SVV-001, at a human biomedical research centre located in the US East Coast region, which was speculated to be due to use of animal products, such as bovine serum or trypsin of porcine origin, in cell culture. Yet, a retrospective study conducted later revealed that SVV had circulated in US swine populations as it was infrequently detected in archived clinical specimens dating back to 1988. Interestingly, SVV received great attention in human medicine since its discovery because of its oncolytic property, whereas the virus wasn’t considered significant in the US swine industry until after SVV was detected in pigs with idiopathic vesicular disease.
Clinical presentation
The term swine idiopathic vesicular disease (IVD) was coined to designate sporadic cases in which pigs display fluid-filled vesicles and/or erosions on the snout, lip, oral cavity, tongue, skin, coronary band and interdigital skin with an unknown cause. The occurrence of swine IVD has been described in the US and other countries including Australia, New Zealand and Italy since the early 1980’s. All these cases tested negative for well-known vesicular disease pathogens, such as FMDV, SVDV, vesicular exanthema of swine virus (VESV) and vesicular stomatitis virus (VSV). In 2008 and 2012, case reports of swine IVD in which SVV was detected, but not the conventional vesicular disease pathogens mentioned above, was made from the US and Canada, raising a speculation that SVV may be a causative agent for vesicular disease.
During late 2014 and early 2015, several states in Brazil experienced outbreaks of vesicular disease manifested by cutaneous lesions (hyperemia, vesicles, ulceration) on the snouts and coronary bands of sows and growers. In addition, increased neonatal mortality was observed during the first week after birth in many breeding farms. Some herds also experienced increased incidence of neonatal scouring. Samples taken from clinically affected pigs tested negative for FMDV, SVDV, VESV and VSV; however, SVV was detected by PCR in vesicular fluid, serum and tissues. Starting in the summer of 2015, a rise in cases of IVD, which clinically resembled the cases in Brazil among pigs at exhibitions, packing plants and commercial farms was noted in several states of the US, alarming state and federal regulatory agencies and swine industry professionals due to the concern of foreign animal disease (FAD) or a new emerging disease. Affected animals also showed acute lameness, lethargy and transient fever. In some breeding herds, increased neonatal mortality for a short period was also reported. All of the cases tested positive for SVV but negative for FAD agents to the US. No other microbial agents were consistently identified among the cases. Since the US incidences, vesicular disease cases testing positive for SVV have also been reported in China and Colombia.
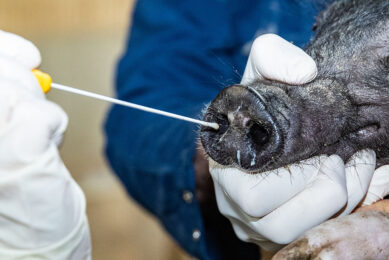
Even though SVV was postulated to be associated with swine IVD, initial attempts to reproduce vesicular disease in pigs under experimental conditions were unsuccessful. Recently, multiple groups in the US have been successful in reproducing vesicular disease after experimental inoculation with contemporary US SVV isolates via oro-nasal routes in 3-week-old pigs, 9-week-old pigs, approximately 150-pound finishing pigs and mature gilts. Under the experimental conditions given in each study, the pigs develop vesicular lesions on snout, coronary band and/or interdigital skin between 2- and 7-days after inoculation, leading to coalescing erosion on the coronary band and snout which heals in 2-3 weeks. Older animals become lame due to interdigital erosion, erosion on the coronary band, deep nail bed bleeding, and cracks in the hooves (see image below).

The inoculated pigs are viremic for 5-10 days and shed the virus in faeces and oral fluids for 3-4 weeks. Tonsil was the tissue with the highest virus load and retained the virus for the longest time, which is in agreement with field observation (6 weeks). The presence of SVV in epidermis with vesicular lesions has been detected using immunohistochemical techniques, proving that the virus is able to induce the cutaneous lesion.
In contrast to vesicular disease, the neonatal mortality syndrome has not been reproduced yet by inoculating SVV to pregnant gilts and sows or newborn piglets although transplacental infection of foetuses by the virus was apparent even when sows at late gestation were exposed to the virus.
Epidemiology and ecology
The origin of SVV is unknown. Reservoir animals have not been identified and pigs are believed to be the natural host of SVV. The presence of antibody cross-reacting with SVV-001in serum samples from non-diseased mice and cows was reported. It is interesting to note that a relatively high percentage of the tested bovine serum samples contained anti-SVV neutralising antibodies, suggesting other farm animals can be exposed to the virus and could serve as a natural host. Diagnostic investigations on farrow-to-wean, farrow-to-finish and sow farms undergoing IVD has demonstrated the presence of SVV in not only swine specimens (lesion swabs, serum, tissues) but also faeces and intestines from mice caught on the farms. Some (1%) of internal (rectal and oral) or external (feet and fur) swabs collected from wild mice captured in Iowa’s wetlands in 2015 were also positive for SVV RNA. These observations suggest that mice may play a role in transmission of the virus within farm or between farms in a close proximity.
The incidence rate of SVV-associated vesicular disease in the field appears to be higher during warmer weather seasons than cold weather seasons; however, the presence of SVV in pig populations is year-round according to diagnostic laboratory data, and no insect vector has been identified.
Interestingly, detection of SVV RNA in flies caught inside and outside of buildings with pigs undergoing SVV-associated disease was reported, suggesting that flies could be at least a mechanical vector contributing SVV spread between rooms within a production facility once the virus is introduced.
Prevalence in the US
It is unknown how prevalent SVV infection/exposure is in US swine populations. In 2015 when increased SVV-associated IVD cases were noted, a ‘quick-and-dirty’ surveillance study was conducted to examine oral fluids submitted to veterinary diagnostic laboratories in Iowa and Minnesota between August 24 and September 1 for SVV. In total, 2033 oral fluids from 441 swine operations without clinical signs of vesicular disease in 25 states were tested by PCR. Approximately 1.2% of the oral fluids representing five swine herds in five states tested positive for SVV RNA, showing a low prevalence of virus infection at the time when an increase of SVV-associated vesicular disease emerged in the US. Thereafter, the virus appears to spread to more farms. Accumulative laboratory testing results for SVV among case submissions to Iowa State University Veterinary Diagnostic Laboratory since June of 2015 show that SVV RNA has been detected in approximately 16.6%, 14.3%, 4.6% and 1.4% of all samples tested in 2015 (July-December), 2016, 2017 and 2018 (January-March), respectively. It should be noted that these data are biased since cases are not stratified by clinical status, herd origin, animal age and sample type for analysis.
Knowing that SVV has been around in US swine for a long time, a sudden uptick of SVV-associated IVD in the US is still a mystery. One of the logical assumptions would be a change in the virulence of virus which may have been associated with genetic changes since earlier attempts to reproduce the disease with ‘old’ strains of SVV failed. However, in recent animal studies conducted at the USDA National Animal Disease Center, both ‘old’ and contemporary isolates of SVV were found to replicate in pigs comparably and cause vesicular disease under experimental conditions even though the virus strains had as high as 7% difference in whole genomic sequences. If there were co-factors missing, further investigations remain to identify these.
Prevention and control
The economic significance of SVV-associated vesicular disease has not been estimated but is not expected to be high because:
a) the virus infection causes no or a very low mortality except neonatal piglets;
b) the disease does not last long and is generally self-limiting; and
c) sows can develop the immunity to SVV and pass lactogenic immunity to newborns. Nonetheless, because vesicular disease associated with SVV infection is clinically similar to other well-known vesicular diseases such as FMD and SVD, there is the cost associated with diagnostics and business interruption particularly related to pig movement during investigation by regulatory personnel. In breeding farms, neonatal mortality can cause a productivity loss even for a short period.
There are no commercial vaccines for SVV available to veterinarians and producers. All strains of SVV known to date, fall into the same serotype, unlike FMDV that has 7 known serotypes. SVV appear to induce good protective immunity against a subsequent challenge, which may last for 5-6 months after initial exposure and humoral immunity seems to be sufficient for disease protection. Therefore, vaccination can be a viable option to consider for prevention and control of SVV. An experimental inactivated whole virus vaccine has shown its efficacy against SVV-associated vesicular disease. Virus-like particles can be a safe and fast way to produce an efficacious inactivated or attenuated vaccine. However, the sporadic nature of SVV-associated vesicular disease and its relatively low economic impact might not justify the cost associated with vaccination.
Biosecurity is an important prevention strategy for SVV. Some risk factors identified with SVV-associated vesicular disease are replacement pigs and delivery or pick-up truck. Therefore, a biosecurity protocol to address these risks, including appropriate testing plan for replacement pigs, should be in place. Feedstuffs should be viewed as a potential source of the virus depending upon storage condition and origin. Vermin control, particularly for rodents, should be implemented reducing the risk of virus transmission. When herds have an outbreak of SVV-associated vesicular disease, infected pigs shed the virus in faeces which is a source for environmental contamination including feeds. The virus is known to survive well in the environment and feedstuffs. Therefore, facilities and equipment should be cleaned and disinfected during and after outbreak.
As is the case for many swine diseases, effective prevention and control for SVV requires appropriate diagnostic testing and periodic monitoring. Pigs on a source farm for replacement animals should be tested for both the virus and antibody. The swine industry must remain diligent for any signs of vesicular disease and report them to authorities for appropriate investigation as viral vesicular diseases can be clinically indistinguishable from each other.