New oedema vaccine gets EU licence
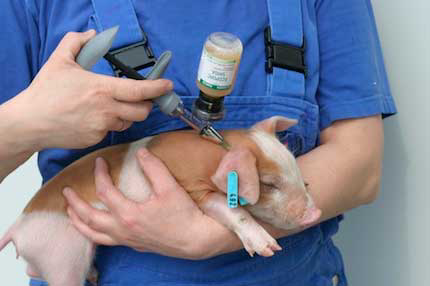
European-wide approval has been granted to Ecoporc Shiga, developed and launched by German vaccine specialists, IDT Biologika the first one-shot vaccine against oedema disease in piglets.
The vaccine provides reliable immunity against this lethal disease estimated to affect 100 million piglets per year world-wide.
“Ecoporc Shiga is now a real alternative to antibiotics and other measures to avoid the threat of outbreaks in nursery pigs,” said Dr Andreas Becker, international marketing manager at IDT.
With the approval from the European Commission, IDT will be organising international distribution of the new vaccine, which has already been licensed in Switzerland.
Shigatoxin-producing Escherichia coli cause oedema disease, resulting in serious economic losses to the pig industry through a high rate of mortality or depressed performance among infected pigs. In individual herds, up to 15% of piglets can die — often the heaviest pigs. Ecoporc Shiga induces a high level of immunity against Shigatoxin (Stx2e, also known as Verotoxin immunity Vtx2e).
Piglets are vaccinated once at four days old to provide proven immunity from weaning and through the entire critical finishing phase. Comparative field studies with Ecoporc Shiga have demonstrated a reduction in mortality due to oedema disease from 11.4% down to 0%.











