PCV2 vaccination changing the pig industry – Part 4. What should be considered when mixing vaccines? Experiences in the US market

Vaccination is a safe and efficacious method of preventing economic damage from an infectious disease. Mixing vaccines for the control of Mycoplasma and PCV2 has become common practice in North America. This paper highlights what needs to be respected with regard to vaccine handling, vaccine preparation and vaccine administration from a practical point of view.
By Dr Tom Gillespie, Rensselaer Swine Services, USA
Vaccination against disease causing pathogens is one primary tool veterinarians and producers use to achieve and maintain herd health. Epidemiology is the study of factors affecting the health and illness of populations which includes the causes, distribution and control of disease. To successfully implement a proper vaccination programme, one has to understand basic elements of the pathogen or pathogens in question. The principal aims of vaccination are threefold:
SBlt To protect the individual animal from the affects of an infectious disease, with associated mortality, morbidity and long term economic sequel,SBlt To prevent or lessen the economic affects of an outbreak of disease in a herd,SBlt Ultimately to eliminate a major pathogen, as in the case of pseudorabies.Vaccination is a safe and highly efficacious method of preventing economic damage from an infectious disease; however, successful vaccination depends upon the following:SBlt Vaccine handling and storageSBlt Preparation of the respective vaccine(s)SBltAdministration technique(s)
Vaccine handling and storage
Even the best ‘high-tech’ vaccine can be negatively impacted by inappropriate handling or storage mistakes. Therefore, some simple rules should be respected when handling vaccines. All vaccines have a predetermined shelf life and expiration dates are clearly marked on the outer packaging of each product. The expiration date is also dependent upon the vaccine being stored in the correct manner. This includes maintaining the product at the proper temperature during shipment, i.e. cold chain, and throughout the storage of the product.
Improper temperature swings during the cold chain may result in loss of potency of the vaccine and could lead to vaccine failure. Most vaccines must be kept at temperatures between 2SDgrC (35SDgrF) and 8SDgrC (46SDgrF). Many vaccines are heat sensitive and may deteriorate if kept at temperatures higher than recommended. Extreme temperatures, such as freezing, will deteriorate the product as well. Some vaccines are also sensitive to direct sunlight; therefore, they are usually packed in amber glass vials and should be kept out of direct sunlight.
Preparation of the respective vaccine(s)
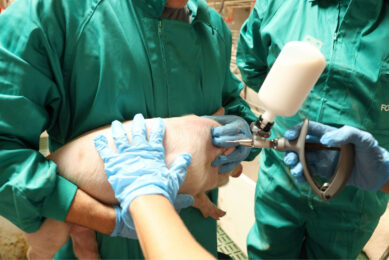
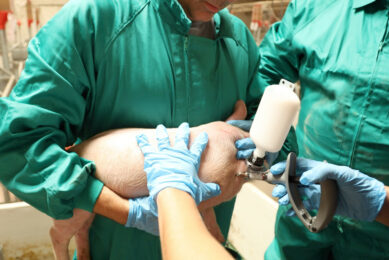
Rehydration of freeze-dried vaccines became common several years ago. Transfer needles are now available to relocate the diluent into the vaccine bottle. It is noteworthy that only sterile equipment should be used when handling injectable products. More recently ‘combinable’ swine vaccines are available. Ingelvac Circoflex and Ingelvac Mycoflex (both manufactured and marketed by Boehringer Ingelheim) are an example of a licensed product for combination (in the US, Canada and Mexico since 2008). The main reason for using combination vaccines is to reduce stress to the animals, especially during the weaning process. In addition, the reduction in workload is greatly appreciated by the producer.
The label for each product is the fundamental guide on how to appropriately use the vaccine with recommendations e.g. route of administration, injection/ application site, indications for use and withdrawal time.
Vaccines should not be combined unless the mixing is supported by the manufacturer and appropriate mixing guidelines are provided. It is pertinent to always read label directions or instructions on how to properly mix two vaccines. Antigen and adjuvant is carefully selected and adjusted, which is done during the development. This process is vital so the vaccine provides optimal immunity.
As a basic rule that mixing of vaccines with different adjuvants should be avoided. Improperly mixing vaccines with different adjuvants can lead to severe efficacy and safety failures, or at least may be one factor why the vaccine did not perform as well as expected.
When preparing a vaccine or combining vaccines, one needs to be sure that the adequate number of doses is prepared. Once a bottle of vaccine is opened, the entire content should be used immediately in order to avoid cross-contamination with bacteria.
Contaminated vaccine can lead to adverse reactions and even death in rare cases. This is the reason why all vaccine manufacturers recommend using the entire contents of the bottle once a vial has been opened.
Six mixing rules:
1. Never mix vaccines unless the mixing is supported by the vaccine manufacturer
2. It is important to read the vaccine label and mixing guidelines
3. Do not mix vaccines with different adjuvants
4. Make sure the appropriate number of doses from each of the combinable vaccines is available
5. Use sterile equipment for the preparation of the vaccine mix
6. Mix the entire contents and use the whole mixture immediately
Injection technique(s)
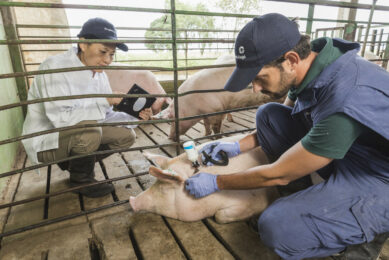
The vast majority of pig vaccines are intended for intramuscular injection. Volumes larger than two ml per injection should be avoided in young pigs. Some vaccines can be injected subcutaneously as well as intramuscularly.
It is important to use only sharp needles and needles that have not been bent during the injection process. Needle size depends upon the size of the pig. A guideline for using the right needle size is given in Table 1.
In case of broken needles, it is pertinent to try removing the broken needle as soon as possible. If the broken needle can not be removed from the animal, the pig and the approximate location of the broken needle must be identified at market time for the packer.After completing the vaccination(s), clean the syringe surface with soap, warm water and an appropriate brush. The inside components are rinsed repeatedly with only hot water and air dried. The use of disinfectants to clean the inside components of the syringe will come in contact with the future use of a vaccine. Disinfectants could neutralise the vaccine or cause adverse problems with the syringe parts. Storage of the syringe and syringe parts must be in a clean and dry environment. Reuseable needles and transfer needles should be cleaned by using boiling water or sterilised by autoclaving.Please remember that only healthy animals should be vaccinated to achieve the best results. Diseased animals do not respond appropriately to any vaccine since they are under disease stress.
The proper use of vaccines is key to achieving success which translates into performance improvements. Using approved combinable vaccines is becoming increasingly popular as it reduces stress for the animals and workload for those administering the vaccine.
Source: Pig Progress magazine Volume 26. 5